Lesion Characteristics
Lesion Size (diameter): 18 mm
Lesion Location: Right Upper Lobe (RUL) lesion
Bronchus Sign: No
Visible on Fluoro: Yes
REBUS Verification: Eccentric Signal
Case Information
Full Procedure Time: 65 minutes
ROSE: Sampling was performed and was suggestive of NSCLC
Final Pathology Report: Final pathology was lung adenocarcinoma
Patient Background
A 72 year-old man with COPD and a smoking history was sent to the pulmonary clinic on finding a right upper lobe (RUL) lung nodule from a low-dose lung screening CT scan performed by his primary care doctor. No prior history of any lung cancer or malignancy. Found to have PET avidity at the lung nodule sent for tissue diagnosis.
The Procedure
Planning
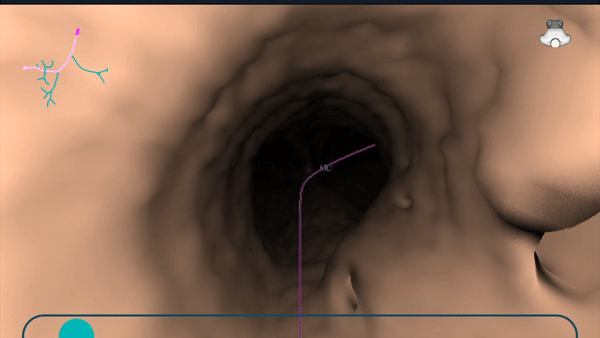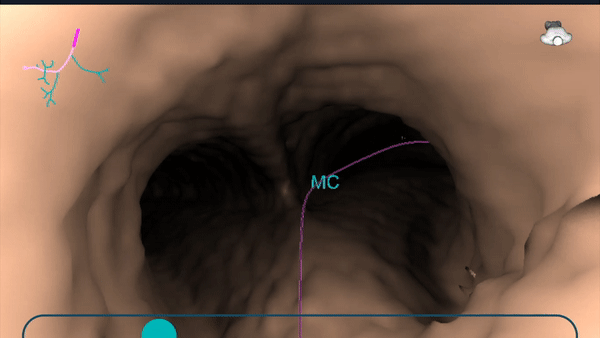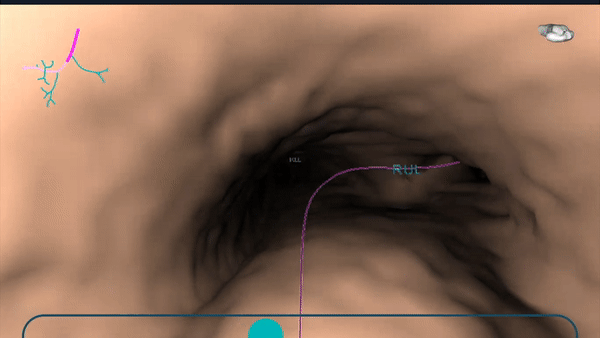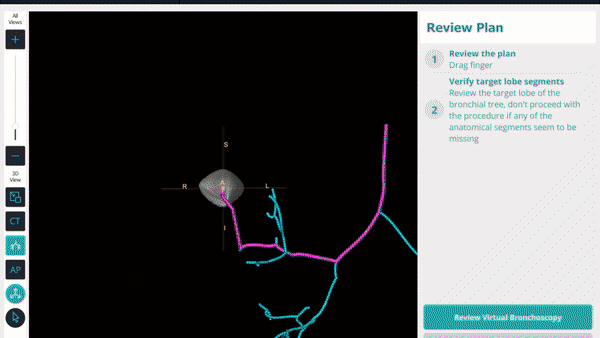
During planning, a virtual bronchoscopy (VB) of the preferred pathway to the lesion is provided. This can be recalled at any time during the procedure to be used in conjunction with the augmented fluoroscopy to assist with navigation to the lesion location.
During planning, a pre-operative CT is uploaded onto the Body Vision system. The lesion was marked and contoured to inform the Body Vision system of the approximate lesion size, shape and location prior to the procedure. The system also derives the airways from the preoperative CT and, during the planning step, a preferred pathway to the lesion is marked so that the Body Vision system can serve up a virtual bronchoscopy to function as a complete standalone navigational bronchoscopy platform.
Registration
Once the patient is intubated on the table, registration of the Body Vision system begins. Registration consists of two C-arm spins. The first spin produces a scan of the main carina and that is marked so that the Body Vision system can use that as a reference point for instructing how to position the C-arm such that it is iso-centered over the lesion.
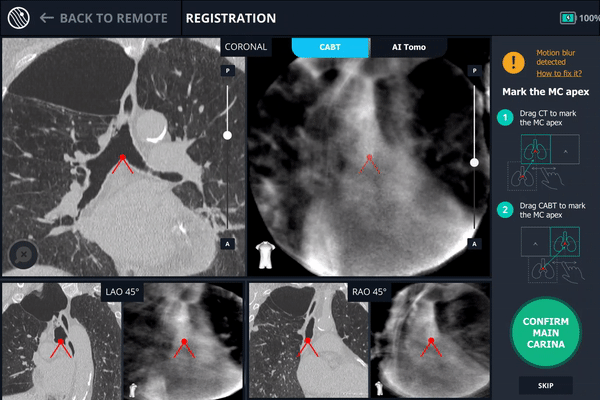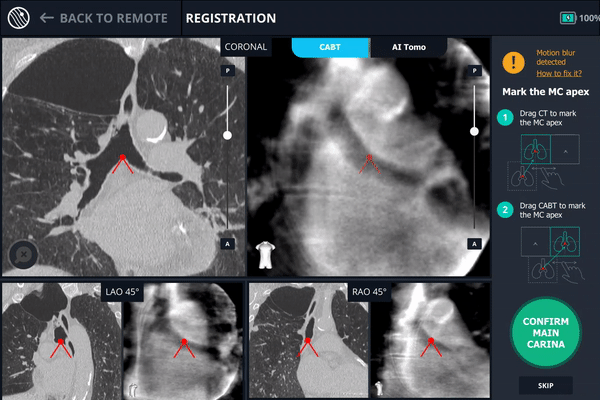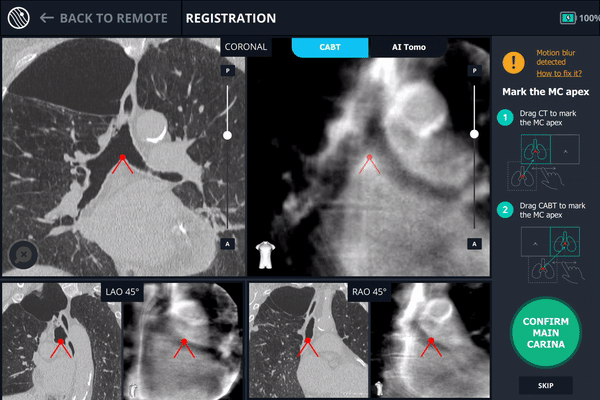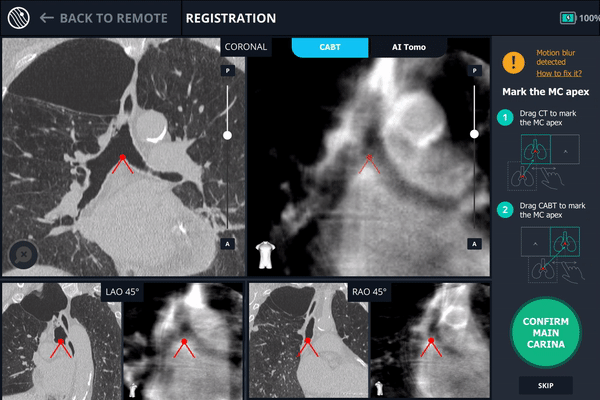
During registration, the main carina is marked on Body Vision’s tomographic 3D scan acquired from the first C-arm spin.
The second spin produces an initial C-Arm Based Computed Tomography (CABT) scan in which the clinician can confirm actual lesion and lesion location before navigation. Registration is now complete.
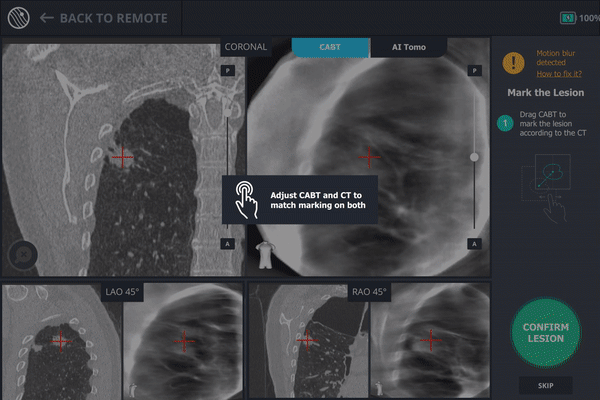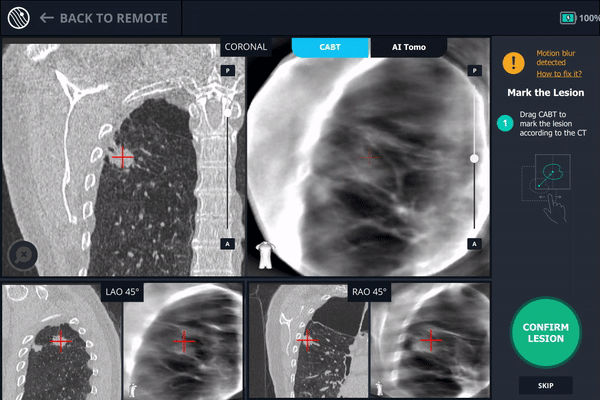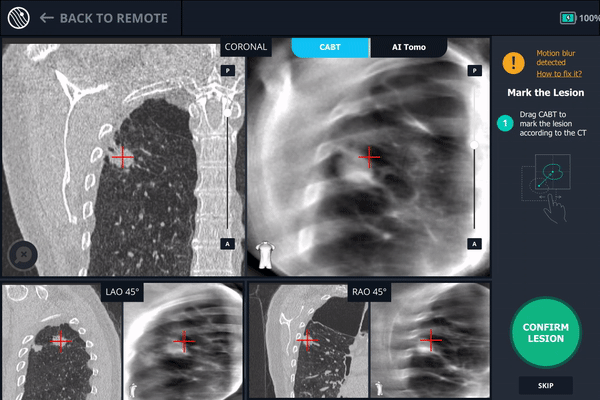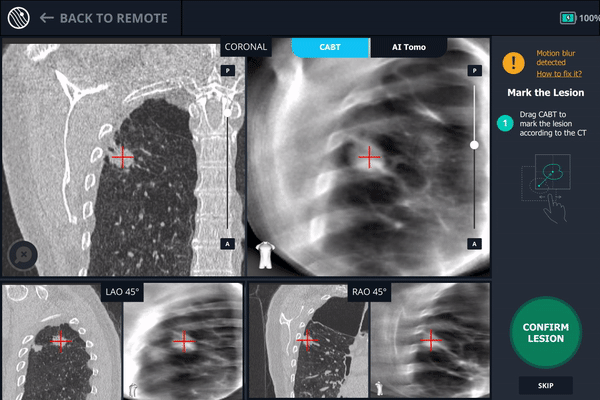
During registration, the lesion is also marked on Body Vision's C-Arm Based Tomography scan so that the system knows roughly the shape, size and location of the lesion and can provide guidance for how to iso-center the C-arm over the lesion during the procedure.
Navigation
During this case, Body Vision was used as an end-to-end navigational bronchoscopy platform. However, for those using a robotic bronchoscopy platform or EMN system to navigate, Body Vision can be easily integrated into that workflow. Using Body Vision's augmented fluoroscopy, the ability to see the bronchoscope in real-time in conjunction with overlays showing lesion location and airways provided the necessary guidance to successfully navigate to the lesion. Body Vision's augmented fluoroscopy is not based on preoperative CT scans and the overlays of lesion location is updated with each C-arm spin. As a result, none of the imaging provided by the Body Vision system are subject to CT-to-body divergence.
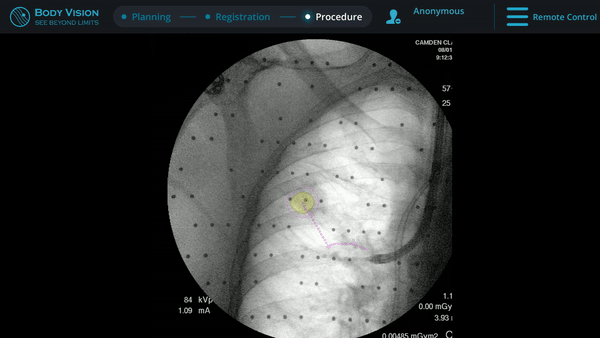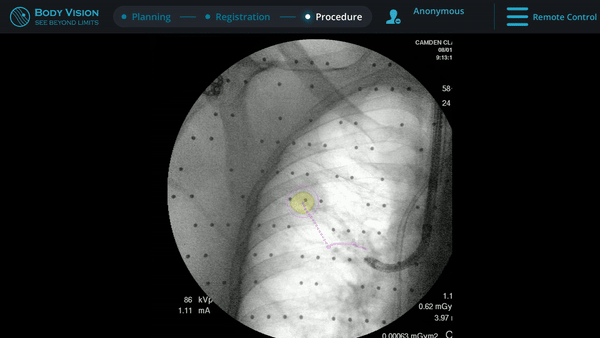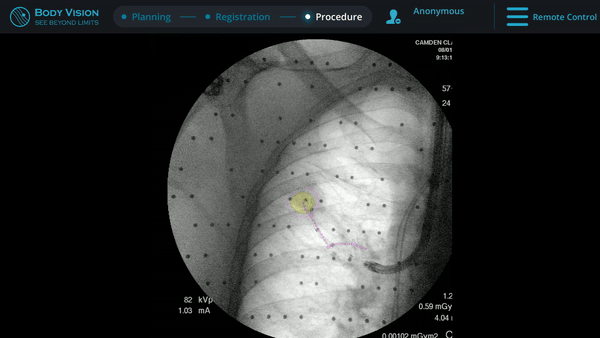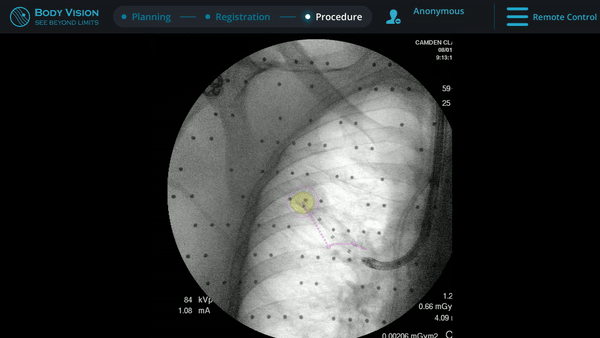
Body Vision’s fluoroscopy augmented with lesion location as visualized on the most recent C-arm spin as well as preferred pathway guidance for real-time, intraoperative navigation.
Tool-in-Lesion Spin #1
Once near the lesion location as seen on augmented fluoroscopy, a C-Arm spin was performed. This spin showed that the tool was anterior to the lesion meaning a repositioning of the bronchoscope was necessary.
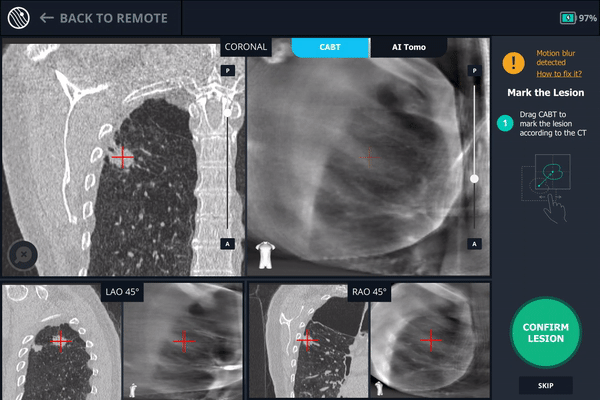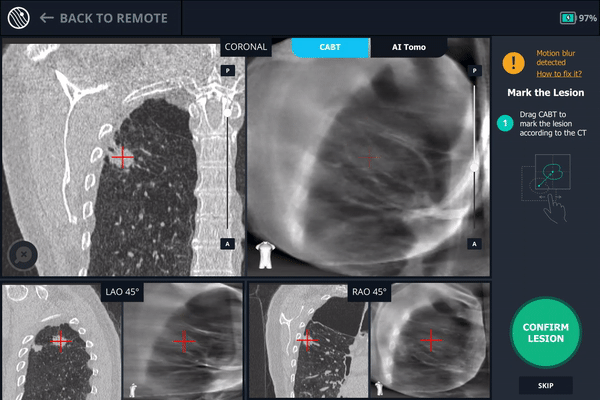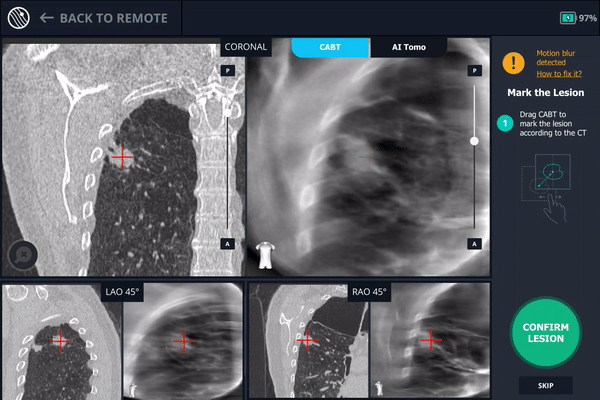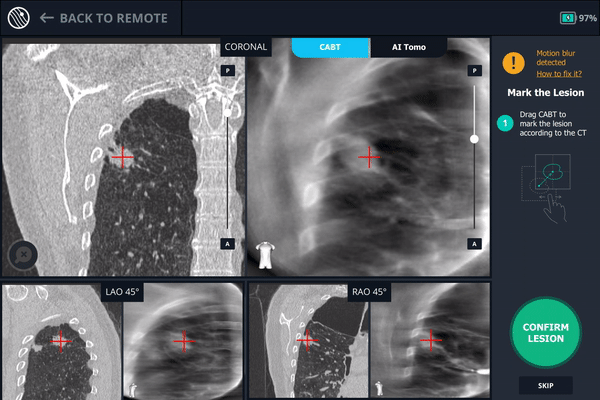
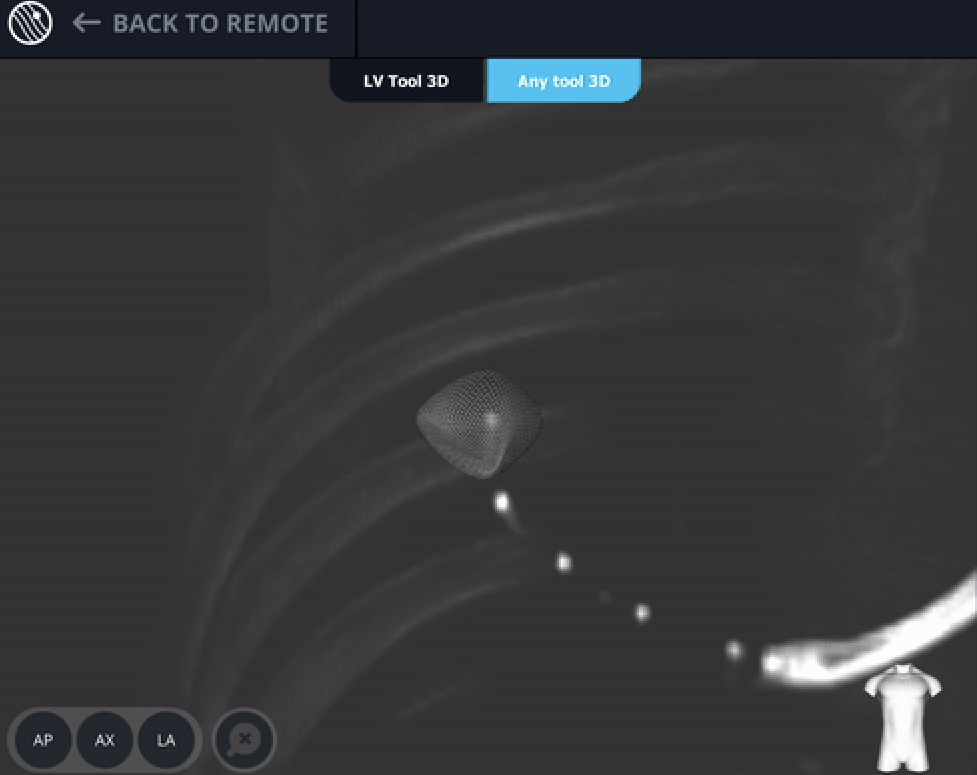
Body Vision's CABT showing lesion location.
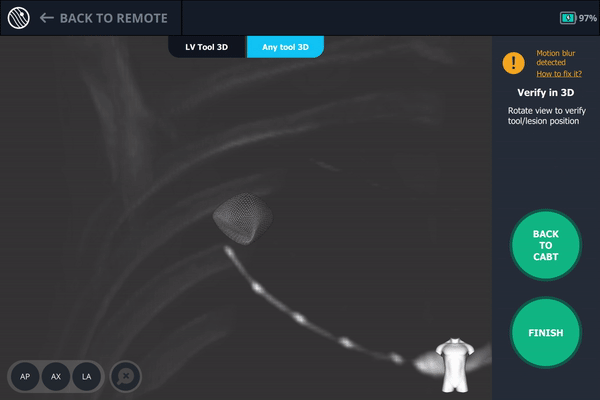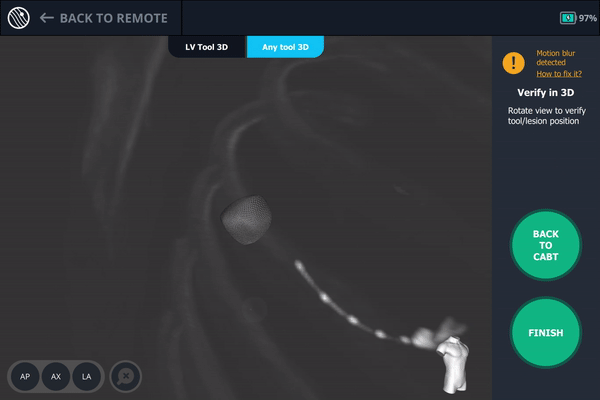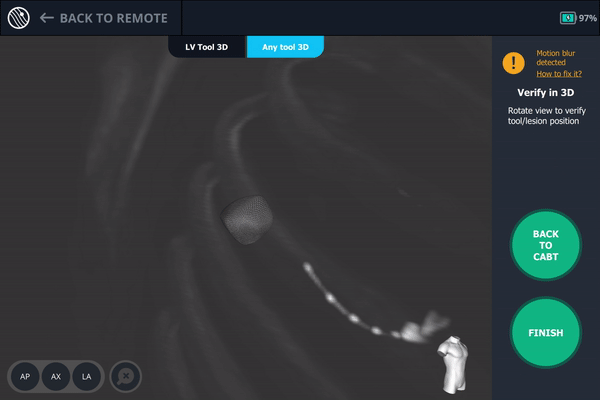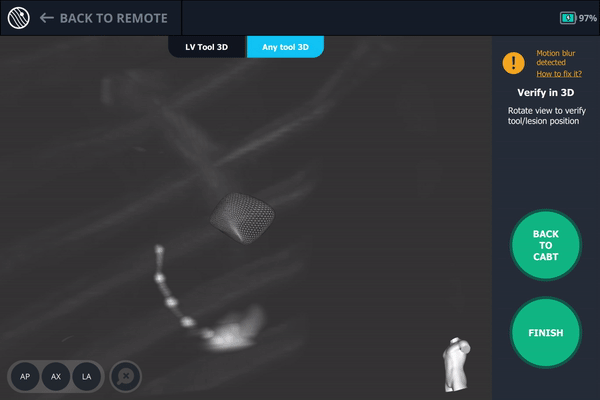
Body Vision's 3D View confirming that the tool was anterior to the lesion.
Readjustment
Using Body Vision's augmented fluoroscopy for guidance, the bronchoscope was repositioned such that it was better angled towards the lesion. This was confirmed by using the C-arm to check the relationship between the bronchoscope and lesion in multiple planes.
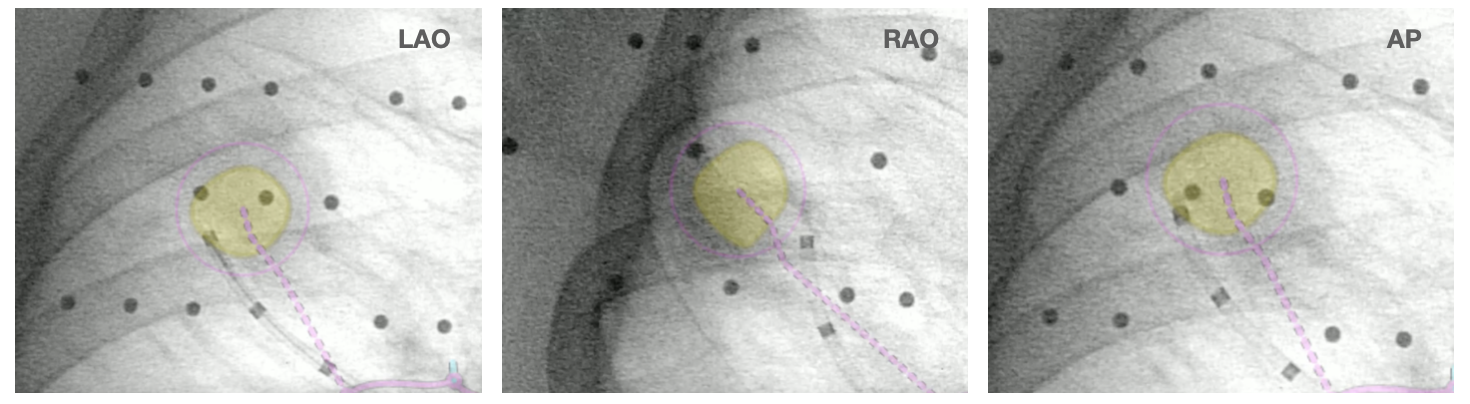 Augmented fluoroscopy in multiple 3D views (RAO, LAO, and AP in this case) also confirmed that the bronchoscope was anterior to the lesion.
Augmented fluoroscopy in multiple 3D views (RAO, LAO, and AP in this case) also confirmed that the bronchoscope was anterior to the lesion.
Tool-in-Lesion Spin #2
Another tool-in-lesion spin was performed after readjustment. The tomographic 3D image provided by Body Vision confirmed that the bronchoscope was better positioned than before, but was now slightly on the posterior edge of the lesion.
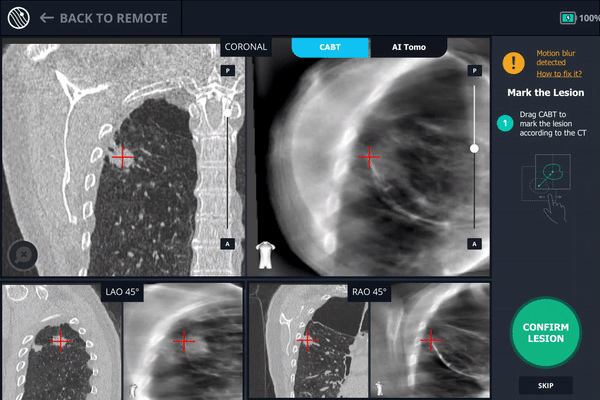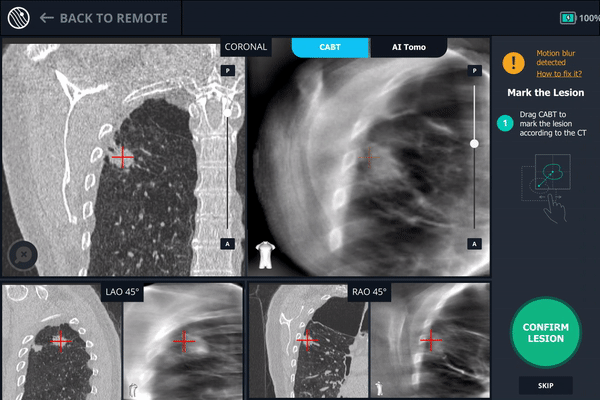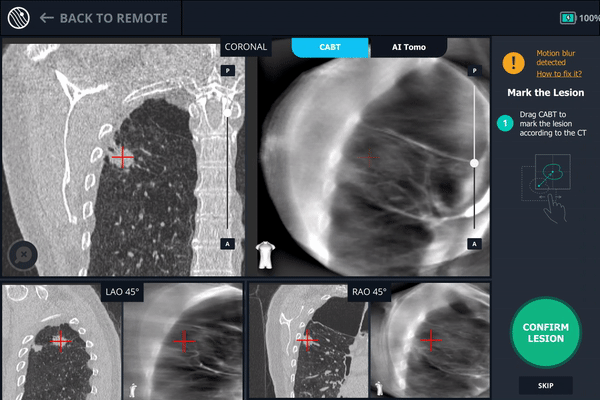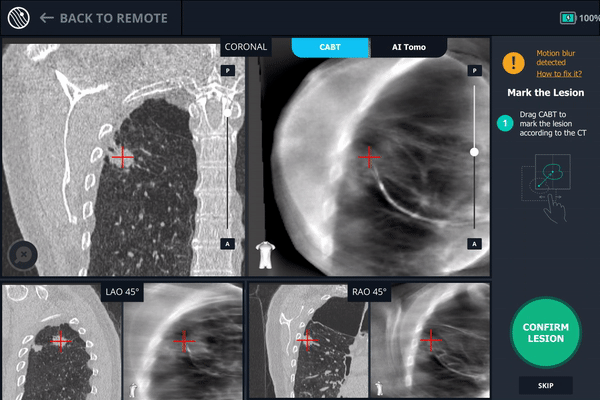
Body Vision's C-Arm Based Tomography providing a tomographic 3D scan of both the lesion and the bronchoscope showing the catheter with biopsy needle extended slightly posterior to the lesion.
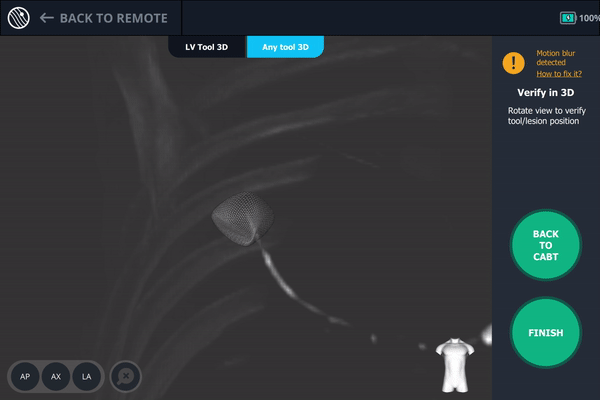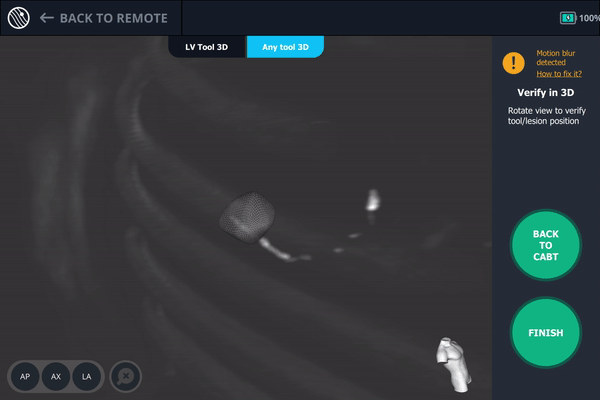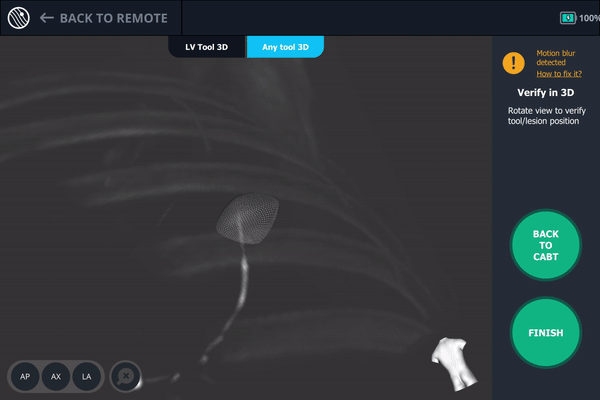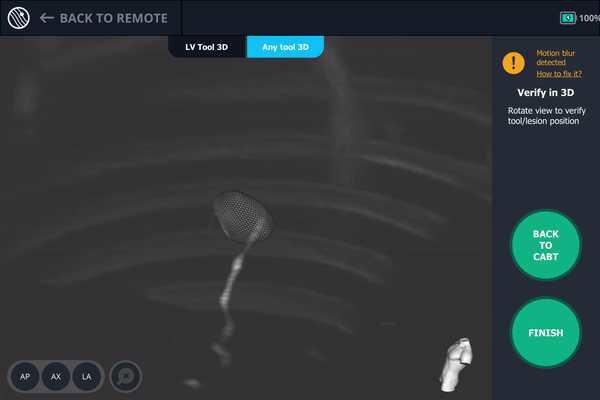
Body Vision's 3D View also confirmed that the bronchoscope was slightly posterior to the lesion.
Tool-in-Lesion Spin #3
A second readjustment was made and a third spin was performed. This spin provided visual confirmation of tool-in-lesion in all three multiple 3D planes.
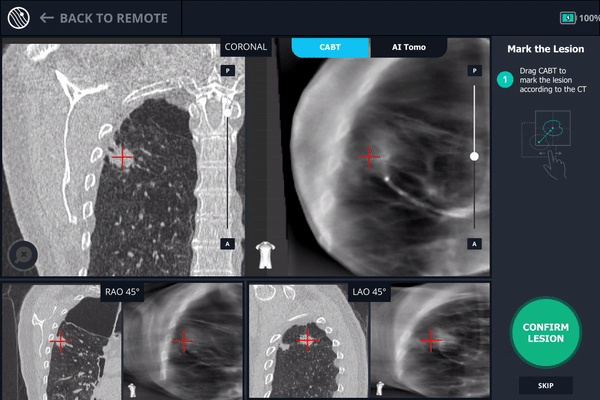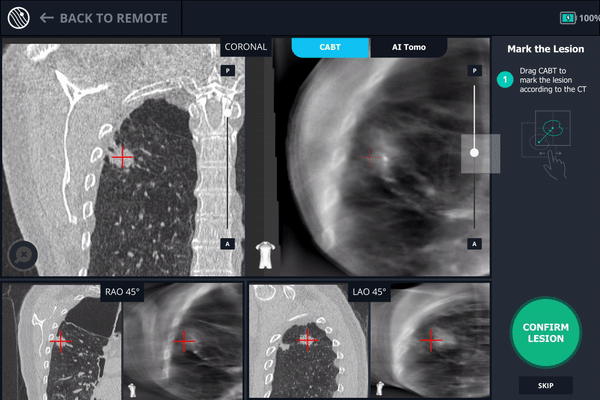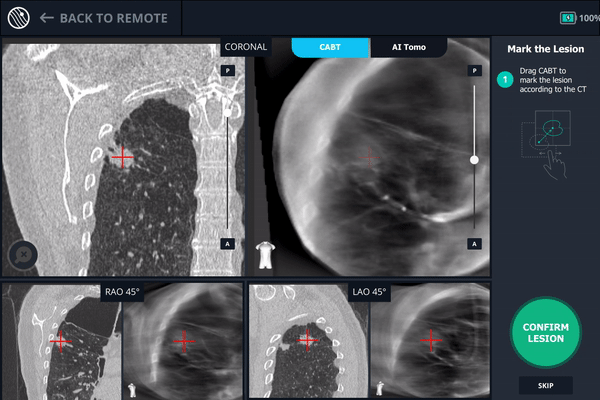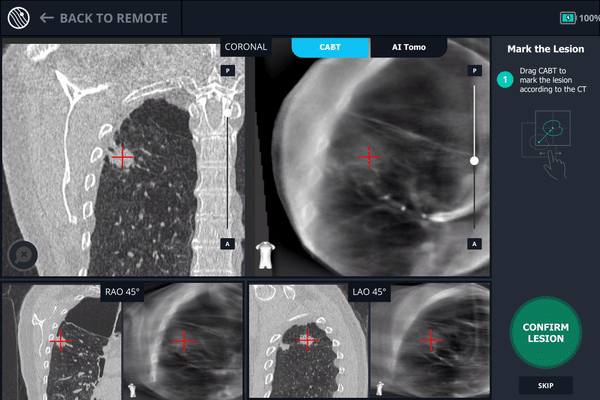
Body Vision’s AI-driven imaging providing tool-in-lesion confirmation prior to biopsy.
Body Vision's 3D View also confirmed that the biopsy needle was right in the middle of the lesion on the third spin.
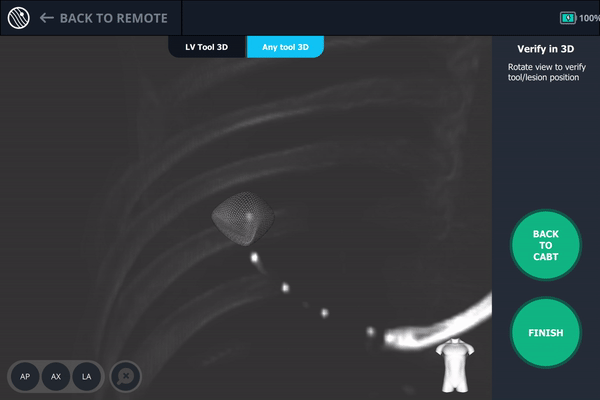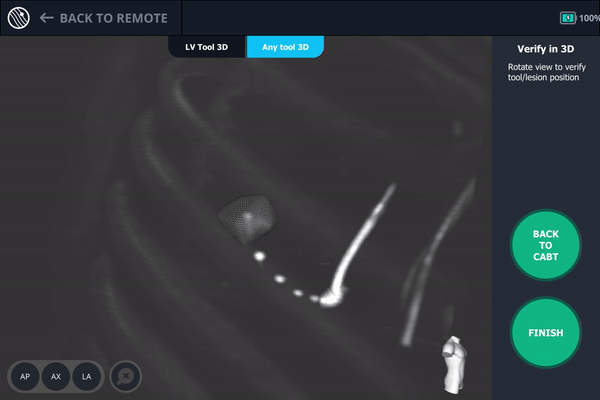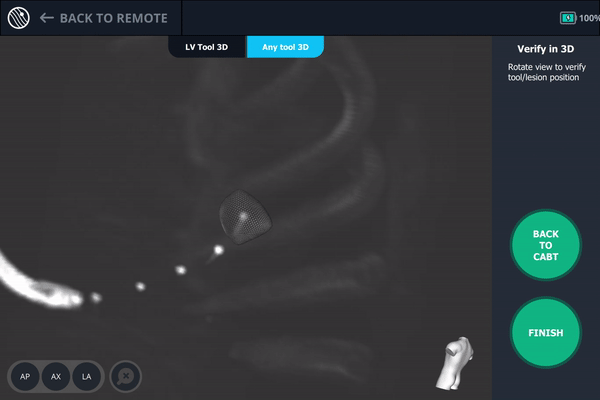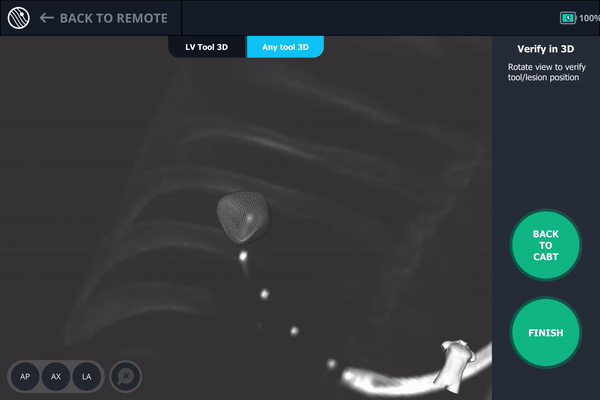
Body Vision's 3D View visually confirming tool-in-lesion.
r-EBUS Confirmation
Once receiving visual, tool-in-lesion confirmation, a r-EBUS probe was inserted down the working channel of Body Vision's procedural kit and returned an eccentric view, further validating that the lesion location shown by Body Vision was the actual lesion location.
Biopsy
Following tool-in-lesion confirmation, sampling of the lesion using a biopsy needle commenced. Body Vision's augmented fluoroscopy provided a real-time view of both the biopsy tool and the lesion to guide how far to throw the needle in order to ensure that the tissue acquired was from within the confines of the lesion. While the Rapid On-Site Evaluation (ROSE) assessed that samples collected showed some atypical cells suggestive of adenocarcinoma, the final pathology report later confirmed a diagnosis of adenocarcinoma. Mediastinal staging procedure was done by EBUS showing only N1 disease at 10R lymph node station also showing adenocarcinoma.
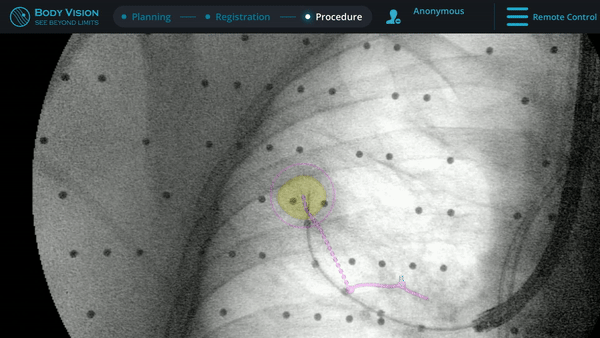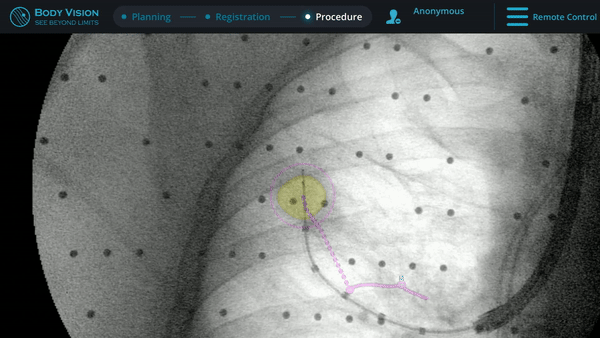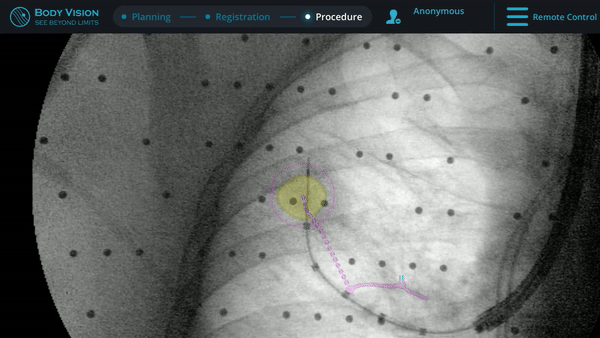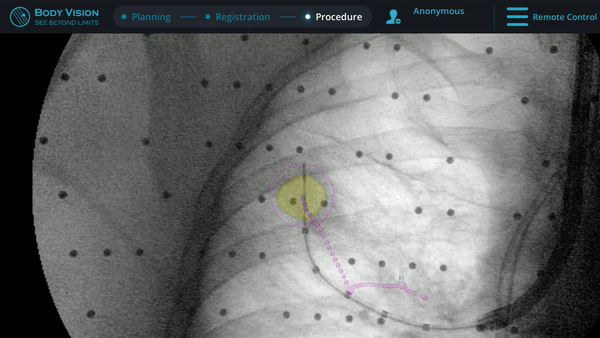
Body Vision's augmented fluoroscopy providing real-time imaging during biopsy.
Conclusion
This case illustrates how Body Vision's AI-driven intraoperative imaging gives physicians the ability to intraoperatively confirm tool-in-lesion prior to and during biopsy. Perhaps even more importantly, since each C-arm spin takes less than a minute and radiation exposure is minimal, if you see that you are not at the lesion, Body Vision’s imaging provides the crucial information needed to reposition the bronchoscope and/or biopsy tools to ensure that biopsy samples are indeed of the suspicious lesion. Because scans are captured during the procedure instead of being reliant on a preoperative CT scan, any CT-to-body divergence is eliminated. Patient is now undergoing treatment with neoadjuvant chemotherapy and plans for a right upper lobectomy.
About Dr. Mathew

Roshen Mathew, MD, DAABIP
Director Interventional Pulmonary / Critical Care
Camden Clark Medical Center