Real-Time, Tool-In-Lesion Confirmation: Inside the Body Vision Process
Following two recent publications in the Journal of Bronchology and Interventional Pulmonology, one in July 2020 and another in October 2020, Body Vision gained interest and inquiries from Pulmonologists and key opinion leaders worldwide. We are often asked, “How is your technology different from Electromagnetic Navigation Bronchoscopy (ENB), and what are the benefits of using Body Vision in my practice?”
These questions are important and have been a key component of our mission since day one. Body Vision is not just a navigation system but also an advanced, real-time, intraoperative imaging modality. Contrary to existing technologies on the market that rely on a pre-operative CT to establish a virtual navigation target and, assuming the bronchoscopist is able to successfully navigate to the lesion, a “cloud biopsy” approach, Body Vision transforms any C-arm into a real-time, intraoperative CT imaging system that enables visualization of the actual lesion during the procedure, thus eliminating CT-to-body divergence and making visual confirmation of tool-in-lesion prior to biopsy a reality. This approach is revolutionizing diagnostic bronchoscopy by providing evidence-based biopsy utilizing equipment that already exists in virtually all bronchoscopy suites.
The main objective of diagnostic bronchoscopy is to get an adequate tissue sample from the area of the suspicious lesion so that a pathologist can definitively determine whether the lung patient has a malignancy. Until our advanced imaging technology, no solution readily accessible to bronchoscopists, empowered them to confidently get to the pulmonary lesion location, confirm their tool was in the lesion prior to biopsy, and provide the real-time visualization required to take an evidence-based approach to biopsy.
Introduction: Definitions and Etiology
Having reliable diagnostic results from a navigational bronchoscopy procedure is essential to improving patient care. A definitive diagnosis achieved via diagnostic bronchoscopy can lead to earlier treatment and greater survival odds. Moreover, achieving a definitive diagnosis greatly improves the patient experience by eliminating the need for additional, potentially more invasive procedures to get a diagnosis and also provides the certainty potential lung cancer patients are seeking.
When navigating diagnostic results, there have historically been two criteria for evaluation:
- Diagnostic Yield - Diagnostic yield can vary based on how specific the criteria is as to what can be considered “diagnostic,” but principally can be defined as the percentage of procedures that yielded a definitive explanation for the presence of the pulmonary lesion, be it benign or malignant.
- Localization - Localization refers to the ability to successfully navigate to a point in 3D space, be that a real or virtual target. Similar to diagnostic yield, typically expressed as a percentage (e.g., percentage of procedures in which the bronchoscopist was able to successfully reach the target).
By the nature of its definition and the current state of navigation technology, high localization is not directly correlated to high diagnostic yield. In a highly dynamic system like the lung, relying on a preoperative CT scan to establish a virtual target creates the very likely scenario that a bronchoscopist can reliably navigate to the virtual target yet not be at the lesion and thus resulting in biopsy samples that do not yield a definitive diagnosis for the lung patient.
Only through real-time, intraoperative imaging where the clinician can visualize the actual lesion and lesion location and, just as importantly, where their bronchoscope and tools are relative to the lesion during the procedure, can a bronchoscopist maximize likelihood of a definitive diagnosis and achieve a high diagnostic yield.
Computed tomography (CT) guided transthoracic needle aspiration (TTNA) has been able to demonstrate a diagnostic yield upward of 92.1%* because interventional radiologists who perform the procedure have access to CT to visualize both the lesion and the aspiration needle during the procedure, enabling them to verify that the needle is in the lesion prior to taking a tissue sample. The lack of similar intraprocedural imaging in diagnostic bronchoscopy is one reason the procedure on the whole has only been able to achieve a comparatively poor diagnostic yield of 72% even when supplemented by electromagnetic navigation. ³
However, CT-guided TTNA has been associated with higher incidences of complications like pneumothorax, so an approach that combines the low patient risk factor of traditional navigational bronchoscopy with the high diagnostic rates and consistency of TTNA would be ideal.
In 2014, Body Vision Medical was founded to meet the unmet clinical need for early, definitive lung cancer diagnosis. Body Vision’s AI-driven, intraoperative imaging combines the low risk associated with traditional navigational bronchoscopy with the clinical outcomes of TTNA by providing bronchoscopists the real-time, intraoperative imaging they lacked through the use of a traditional C-arm. Since its founding, Body Vision has revolutionized bronchoscopy techniques by providing the ability to see the actual lesion and lesion location, which both eliminated CT-to-body divergence as well as providing the real-time, tool-in-lesion confirmation needed to ensure that tissue samples are acquired from within the lesion to maximize likelihood of a definitive diagnosis.
In this blog, we will do a deep dive on the Body Vision technology and how this advanced technology empowers clinicians to maximize diagnostic yield. First, it is essential to understand key terms and definitions related to this process.
- Bronchoscopy - Bronchoscopy is a procedure that allows doctors to look at a patient's lungs and airways. This procedure involves the insertion of a thin tube through the nose or mouth that leads to the throat and lungs. Bronchoscopy is usually performed by a specialist doctor looking for lung disorders or cancer. The doctor can look for abnormal areas in the airways to identify spots that need further consideration.
- Pulmonary Lesion - A pulmonary lesion or nodule is an abnormal growth that forms in a lung. Lung nodules can be either benign (noncancerous) or malignant (cancer). The most common causes of benign nodules include granulomas (clumps of inflamed tissue) and hamartomas (benign lung tumors). The most common cause of cancerous or malignant lung nodules includes lung cancer or cancer from other regions of the body that has spread to the lungs (metastatic cancer). Accurate testing, often via tissue sampling, is required to definitively diagnose whether a lesion is benign or malignant and the cause of lesion.
- Fluoroscopy - Fluoroscopy is an imaging technique that uses X-rays that pass through the body to generate continuous, real-time images of the inside of the body, much like an X-ray movie.
- Computed Tomography (CT) - A computed tomography (CT) scan combines a series of X-ray images taken from different angles around your body and uses computer processing to create cross-sectional images (slices) of the bones, blood vessels, and soft tissues inside your body. CT scan images provide more-detailed information than plain X-rays do. CT scans are used in various diagnostic tests to look for diseases, cancers, and more.
- Transthoracic Needle Aspiration (TTNA) - TTNA is an image-guided biopsy approach used to diagnose thoracic diseases. TTNA uses computed tomography (CT) or ultrasound (US) to provide real-time image guidance as a biopsy needle is introduced through the chest wall into the thorax to take tissue samples of a suspicious lesion. Historically, TTNA has a very high diagnostic rate upwards of 90% but also suffers from a high complication rate of pneumothorax (collapsed lung) close to 25%. ²
- C-Arm Based Tomography™ Technology - Body Vision’s CABT™ technology uses a proprietary artificial intelligence (AI)-driven computer imaging algorithm to transform 2D X-ray images from any C-arm into 3D intraoperative CT scans that show both the actual lesion and lesion location in real-time during the procedure, enabling accurate navigation to the lesion and providing visual confirmation of tool-in-lesion prior to and during biopsy.
The Development of Body Vision’s Tool-in-Lesion
Body Vision was born out of a need for imaging technology that empowers bronchoscopists to deliver diagnostic yields on par with their interventional radiology counterparts while addressing the quadruple aim of healthcare by enabling existing medical staff to deliver superior clinical outcomes and patient experience at a lower cost. Prior to Body Vision, all innovations in pulmonology were in the navigation space. One of the leading technologies in navigation bronchoscopy was electromagnetic navigation bronchoscopy (ENB). Though this technology provided a significant improvement in the ability to accurately navigate to a virtual target, its reliance on a pre-operative CT to set that virtual target made it susceptible to CT-to-body divergence. CT-to-body divergence refers to the difference between the pre-operative CT and where the lesion actually is during the bronchoscopy procedure. In order to address this issue, the Body Vision team turned to a combination of intraoperative CT imaging and augmented fluoroscopy.
Fluoroscopy was a smart choice because it’s widely used in imaging during biopsy procedures and utilizes a commonly available piece of X-ray equipment called a C-arm. Fluoroscopy uses X-rays to continuously take images of the body, providing true real-time imaging. The challenge with using fluoroscopy was that pulmonary lesions are typically hard to see or altogether invisible under fluoroscopy. In theory, since the C-arm is an X-ray device like a CT scanner, it seemed possible to use a computer algorithm to fuse the 2D X-ray images taken from different angles into an accurate 3D scan as a traditional CT scanner would. However, the C-arm is less precise than the CT scanner. Moreover, CT scanners come in many different models from multiple manufacturers, each with differing configurations.
To overcome these challenges, Body Vision developed a computer imaging algorithm driven by Artificial Intelligence. As the Artificial Intelligence system was fed more clinical imaging data, it learned to recognize borders of the lesion, bronchoscopic tools, anatomical landmarks, and other key aspects of the image that ultimately improved image quality that could be derived from a C-arm. This technology for tomographically visualizing radiolucent lesions with a C-arm was coined C-Arm Based Tomography (CABT™). Because the innovation was software-based, Body Vision’s solution is hardware-agnostic and designed to integrate easily into procedural rooms as well as interface with existing bronchoscopic equipment.
The Body Vision System
The Body Vision system was developed not only to ensure navigational accuracy and maximize diagnostic yield by enabling visual confirmation of tool-in-lesion during biopsy, but also to integrate seamlessly into procedure rooms. The system design is sensitive to the fact that procedure rooms are often crowded with hardware and electronic equipment that take up valuable space. Because of this, the Body Vision system has a small footprint and interfaces with existing bronchoscopy platforms, C-arms and other equipment without the need for ancillary equipment beyond the Body Vision itself.
The LungVision™ system is comprised of the following parts:
- Main Unit - The system’s main unit is the core of the LungVision™ system and performs the image processing needed to transform a traditional 2D C-arm into a real-time, intraoperative CT imaging system. The form factor was specifically chosen for its small footprint so that it can be as innocuous as possible in the bronchoscopy suite.
- Tablet - The LungVision™ tablet enables clinicians to wirelessly control the system and manage the procedure from anywhere in the room.
- Positioning Board - A positioning board with three layers of radiopaque beads embedded within it that is placed underneath the patient during the procedure. This enables the LungVision(™) system to readily identify with a single fluoroscopy image as to where things are in 3D space and, because the system is entirely imaging based, the positioning board contains no sensors or electronics.
- Disposable Procedural Kit - For those looking to use Body Vision as a complete, end-to-end diagnostic bronchoscopy platform, the disposable Body Vision procedural kit provides an extended working channel that enables greater maneuverability, visibility of the catheter under fluoroscopy, and access to the periphery of the lung with a traditional manual catheter setup. The lack of any sensors or cameras maximizes flexibility as it won’t interfere with metal, and has the added benefit of making the procedural kit comparatively economical.
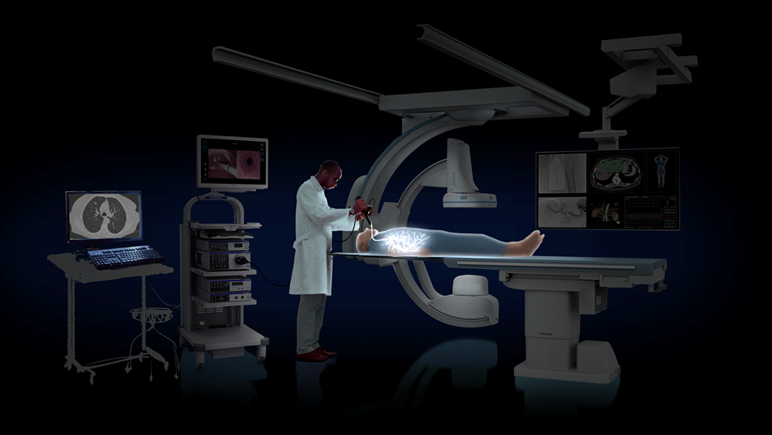
The Procedure
If a suspicious pulmonary lesion is identified during screening, a biopsy is typically required for a definitive diagnosis. More and more, diagnostic bronchoscopy is the preferred method due to its minimal invasiveness and low complication rate. It is in this pursuit of adequate tissue acquisition so that pathology can make a definitive determination of whether the lung patient has cancer that Body Vision’s LungVision™ system comes into play. The Body Vision procedure consists of five steps:
1) Pre-Procedure Planning
2) Registration
3) Navigation
4) Tool-in-Lesion Confirmation
5) Image-Guided Biopsy
1) Pre-Procedure Planning
The first step is pre-procedural planning. A pre-operative CT is uploaded into the Body Vision system. The system derives the airways from the pre-operative CT as well as uses the physician’s marking of the lesion and lesion boundaries to approximate lesion location prior to the procedure so that the system can provide guidance for iso-centering the C-arm over the lesion during the procedure. Here are the steps to planning the optimal pathway:
1) Preoperative CT is uploaded
2) The lesion is identified on the preoperative scan, and the lesion and lesion boundaries are marked.
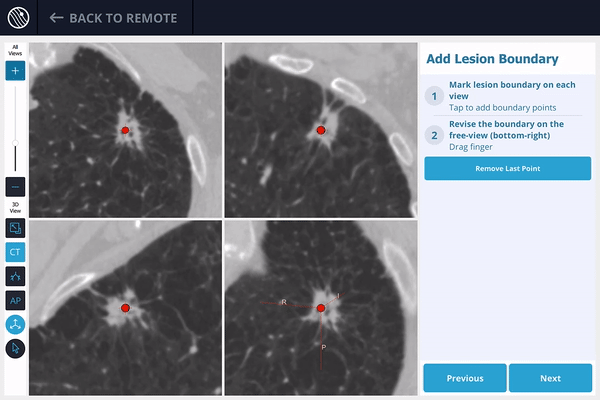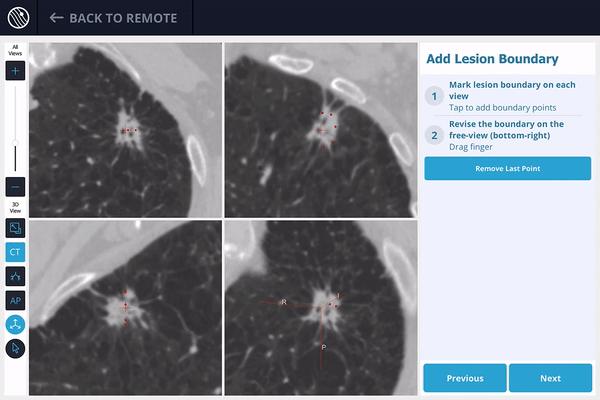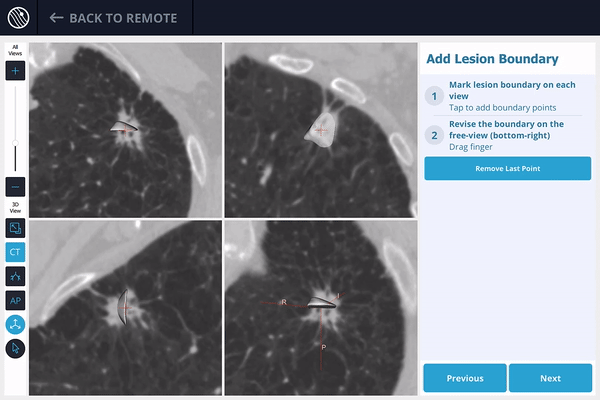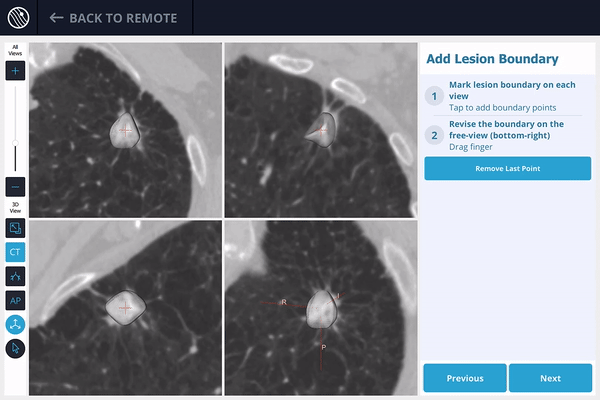
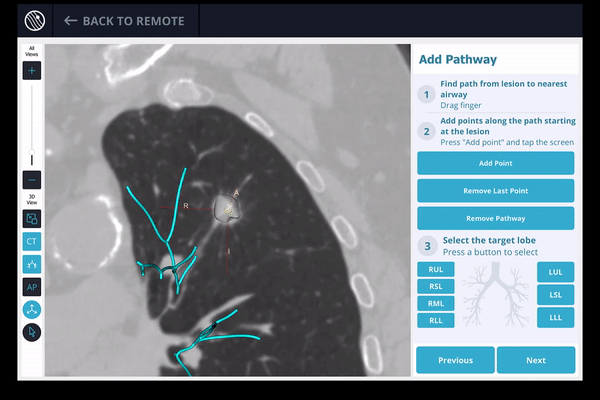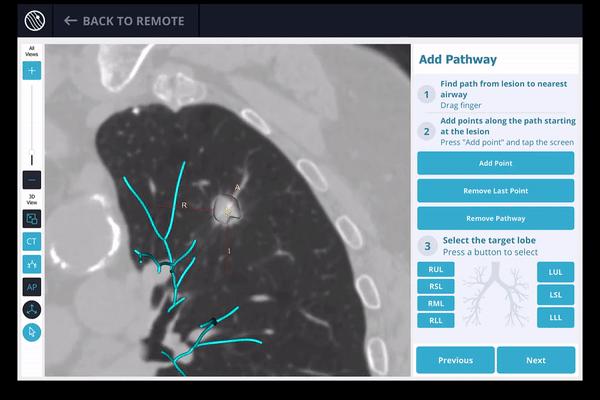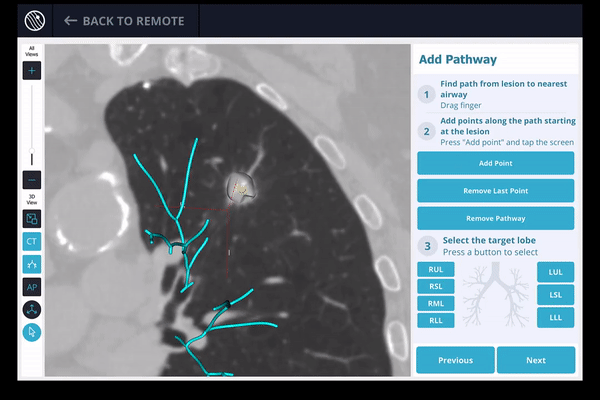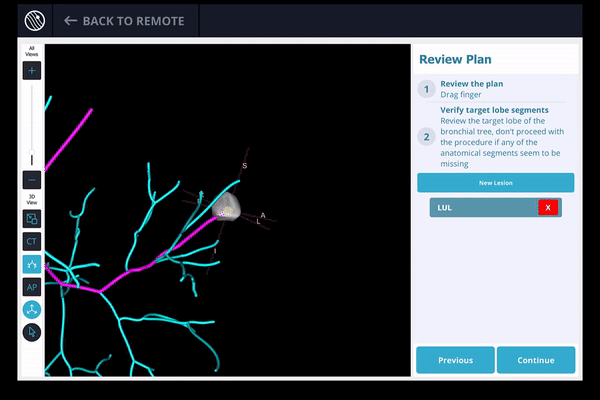
3) The LungVision™ System derives airway information from the pre-operative CT and displays the bronchial tree such that the clinician can choose the preferred pathway, with LungVision™ highlighting the pathway from the entrance of the trachea to the lesion.
4) Once the pre-procedure planning is complete, a virtual bronchoscopy simulation is provided as a summary of the preferred pathway to the lesion.
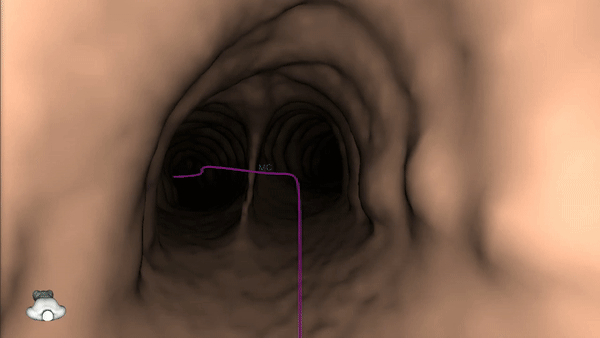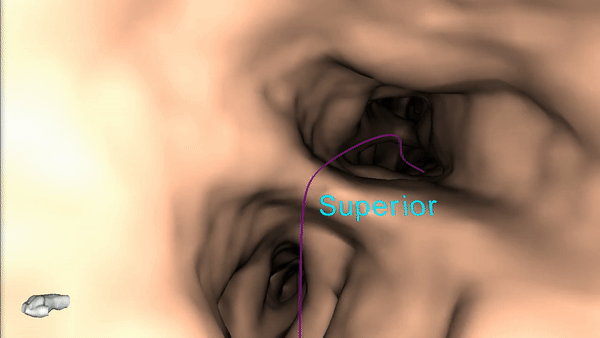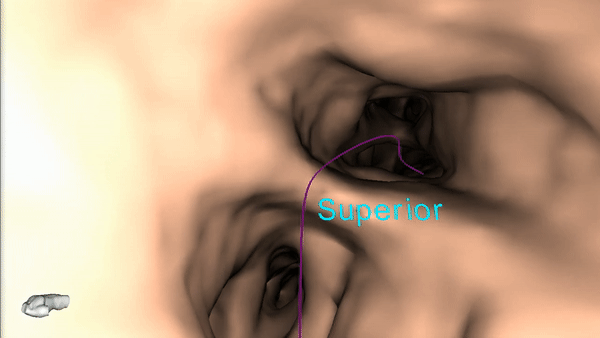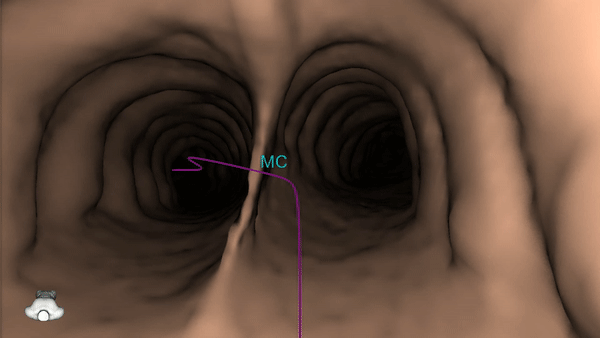
5) The procedure is ready to begin.
2) Registration
Using any conventional C-arm, Body Vision's C-arm-based computational tomography (CABT) technology provides intraoperative 3D images. Registration of the Body Vision system entails a CABT scan of the main carina and a second CABT scan iso-centered around the lesion which provides visualization of the actual lesion and lesion location, even of radiolucent lesions which are typically not fluoroscopically visible, prior to navigation.
3) Navigation
The actual lesion and the pre-planned pathway are displayed as overlays under augmented fluoroscopy. If needed, the virtual bronchoscopy simulation that was shown at the end of the planning step can be accessed at any time during the procedure to provide a bronchoscopic view of the preferred path to the lesion. Once the bronchoscope is driven far enough out into the periphery such that its outside diameter is larger than the airways leading to the lesion, the disposable LungVision™ Procedural Kit is used to extend the working channel and provide the level of maneuverability needed to navigate to the lesion location. Under real-time, augmented fluoroscopy which highlights the lesion location, preferred pathway, and adjunctive airways, the clinician guides the distal tip of the LungVision™ sheath to the actual lesion. Radiopaque markers on the LungVision™ sheath make identification of the sheath and sheath tip obvious under fluoroscopy to aid navigation.
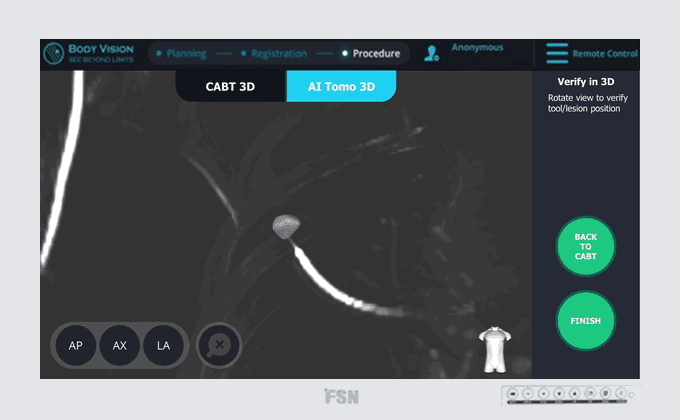
4) Tool-in-Lesion Confirmation
Once the LungVision™ sheath is at the lesion location, it’s time to insert a biopsy tool. Before the sampling, it’s recommended to confirm that the biopsy tool is visually inside the lesion in multiple 3D planes with Body Vision’s CABT™ technology. With CABT™, the user can see the spatial relationship between the lesion and the biopsy tool tip. The interactive 3D View functionality enables Body Vision to present this data as a 3D reconstruction way so that those who have difficulty interpreting the 3D scans in three separate orthogonal planes can determine whether they have achieved tool-in-lesion and, if not, how to position the bronchoscope and/or sheath appropriately in order to get a tool-in-lesion confirmation. With this approach, the question of accuracy and localization become obsolete because the clinician can visualize the actual lesion. There also is no CT-to-Body divergence because Body Vision enables the clinician to see the actual lesion intraoperatively with a conventional C-Arm.
LungVision™ technology is highly compatible and provides physicians with the flexibility to use any instrument of their choice. If desired, physicians may choose to extend a Radial EBUS probe (r-EBUS) down the LungVision™ sheath before inserting the biopsy tool in order to provide a secondary means of confirming lesion location.
5) Image-Guided Biopsy
Once the tip of the biopsy tool is confirmed to be inside the lesion in multiple 3D planes, biopsy under augmented fluoroscopy is performed so that the clinician can verify in real-time that samples are taken from within the lesion. With LungVision,™ the biopsy process is image-guided and evidence-based. There is no longer any need to systematically sample from a wide area around a virtual target in hopes of obtaining adequate tissue samples from within the lesion because LungVision enables visualization of the actual lesion. The LungVision™ procedural kit is compatible with any off-the-shelf, biopsy tools with an outer diameter of 1.9mm or less.
Learn More about the Innovative Body Vision System.
The Benefits of AI-Driven, Intraoperative CT Imaging
Body Vision’s AI-driven, intraoperative CT imaging technology was developed to maximize diagnostic yield and improve clinical outcomes so that lung patients can receive the certainty they are seeking and, if needed, receive timely treatment. Let’s take a look at some of the benefits of using this technology:
- Treat Lung Cancer Earlier - Lung cancer is the second most common cancer in the United States and represents 25% of all cancer deaths annually, more than colon, breast, and prostate cancers combined. The American Lung Association (American Lung Association, n.d.) estimates that 70% of lung cancers are found at a later stage, explaining why, despite improved medical technology, smoking cessation in the population and lung cancer screening, 5-year survival remains at 20%. More prevalent lung cancer screening of high-risk populations in combination with a greater ability to definitively diagnose patients at an earlier stage will enable a higher percentage of lung cancer patients to receive treatment earlier, improving long-term survival.
- Seamless Integration - The Body Vision system was designed for easy integration into existing procedural rooms, bronchoscopy setups, and workflows.
- Use of Artificial Intelligence - Body Vision Medical has pioneered the use of Artificial Intelligence in navigation bronchoscopy. Harnessing the power of AI, Body Vision has developed a solution that enables existing medical teams to deliver superior clinical outcomes and patient experience at a lower cost. Compared with hardware-intensive solutions such as robotics and cone-beam CT, Body Vision’s solution is predominantly software-based which is not only cost-effective, but provides a foundation for the potential rapid iteration of features and functions that have a direct impact on both user experience and clinical outcomes.
The Benefits of Body Vision's Tool-In-Lesion | Case Study
Body Vision’s LungVision™ AI-driven, intraoperative CT imaging system has set itself apart from other technologies due to its ability to maximize diagnostic yield while leveraging existing equipment to make the solution cost-effective. For decades, successfully navigating to and confidently biopsying from peripheral lung lesions was a challenge because bronchoscopists could not see the lesion let alone visually confirm tool-in-lesion prior to biopsy.
Body Vision’s solution addresses this by enabling physicians to visualize peripheral pulmonary lesions in real-time during the procedure, eliminating CT-to-body divergence and providing the ability to visually confirm tool-in-lesion prior to biopsy. There are multiple clinical studies and data in peer-reviewed literature that supports the idea that real-time, intraoperative 3D imaging is key for unlocking higher diagnostic yield regardless of navigation modality. To see how our technology has translated to real results, let’s take a look at one of our case studies. If you want to find more information about this case, you can read more here
https://bodyvisionmedical.com/articles/crozer-health-whitlark-use-case.
Here are some of the key findings from the case study:
Lesion Characteristics:
Lesion Size (diameter): 12 mm
Lesion Location: Right upper lobe (RUL) lesion
Bronchus Sign: No
Visible on Fluoro: No
REBUS Verification: Yes, after Body Vision enabled navigation to the lesion.
Case Information
Full Procedure Time: 45 minutes
ROSE: Sampling was performed and was positive for non-small cell lung cancer (NSCLC)
Final Pathology Report: Final pathology was adenocarcinoma of the lung.
The patient in this case was a 69-year-old asymptomatic current smoker with chronic obstructive pulmonary disease (COPD). This patient underwent a routine CT screening, and doctors found a suspicious nodule in the right upper lobe. A positron emission tomography (PET) scan confirmed hyper-metabolic activity. So, a robotic-assisted navigation bronchoscopy procedure that used the Auris Monarch platform and Body Vision system was scheduled for a peripheral lung nodule biopsy. Here is a look at that procedure:
- Planning - First, a preoperative CT was uploaded to the Body Vision imaging system driven by AI. Then the lesion was marked and contoured so the system could estimate the lesion’s shape, size, and location.
- Registration - Once the patient was on the table, the Body Vision registration was performed using two C-arm spins. One of the spins centered around the main carina, and the other was iso-centered on the lesion. As a result, this produced a CABT™ scan that confirmed the lesion and its location before navigation.
- Navigation - Next, the Auris Monarch bronchoscopy platform was navigated to the virtual target. The radial probe endobronchial ultrasound (r-EBUS) advanced down the Monarch bronchoscope to find the virtual target location. This method did not return an indicative sign of the lesion at the target location, so a C-arm was brought in in conjunction with a CABT™ scan with the Body Vision system. This determined the true lesion location. There was no CT-to-body divergence thanks to Body Vision’s imaging technology which is not based on preoperative CT scans. Instead, it’s captured in real-time.
- r-EBUS Confirmation - Next, the r-EBUS probe was re-inserted through the Monarch robotic bronchoscope channel and then extended into the lesion location that was identified by Body Vision. Once the probe returned, it confirmed the location as determined by Body Vision.
- Tool-In-Lesion Confirmation - The r-EBUS probe was retracted from the channel and replaced by biopsy forceps. These forceps extended into the pulmonary lesion and visualized by Body Vision and verified by r-EBUS. Another C-arm spin was used to give visual confirmation of the tool-in-lesion in multiple 3D planes. Then, the biopsy could be performed.
- Biopsy - The biopsy was performed under real-time imaging provided by Body Vision’s augmented fluoroscopy. This ensured tissue sampling occurred within the lesion. On-site evaluation confirmed that the samples collected were consistent with non-small cell lung cancer. These results were later confirmed using cytology and through a final pathology report.
This case demonstrated the ability of Body Vision’s Artificial Intelligence imaging to incorporate into a historically used diagnostic bronchoscopy workflow seamlessly. The robotic-assisted technology did add stability to the navigation. However, without real-time 3D imaging, it was difficult to make corrections. In this case, there was a correction needed for the Monarch system.
Instead of using blind sampling of the lung using r-EBUS, Body Vision allowed this team to visualize the lesion and its location. In doing so, the bronchoscope was repositioned to the actual location, which enabled tool-in-lesion confirmation. This maximized the chances that the tissue samples were within the lesion and provided a definitive lung cancer diagnosis for the patient. With accurate results, the team could proceed with proper treatment and care. This is just one of many case studies on how Body Vision has transformed the way diagnostic bronchoscopy can be performed accurately on the very first try.
Conclusion
It is undeniable that a novel approach was needed in order to address the problem of how to increase diagnostic yield of navigational bronchoscopy. While there have been innovations in the field of pulmonology in the form of electromagnetic navigation and, more recently, robotic bronchoscopy platforms and cone-beam CT imaging, these solutions either only partially address the problem or provides a solution that are too cost prohibitive, workflow intensive, or access-limited to be viable for a majority of bronchoscopists. Body Vision’s aim of saving lives through the democratization of innovative medical technology led to the development of the LungVision™ AI-driven, intraoperative CT imaging platform which addresses the unmet clinical need for early, definitive lung cancer diagnosis and, in the future, to enable effective treatment of lung lesions via a minimally-invasive procedure.
These are encouraging results, but this doesn’t mean we aren’t still looking to improve the user experience and our customer training. We want our users to achieve autonomy even sooner, so they can focus on providing the best patient care possible. In addition, we want to improve imaging quality. One of the benefits of our technology being software driven is that it allows us to make continuous improvements.
If you are considering an advanced bronchoscopy system for your practice, be sure to read our blog, Determining Success of a Navigation System, where Body Vision partner, Dr. Michael Pritchett, shares the unmatched level of confidence that our platform provides.
- American Lung Association. (n.d.). What to Expect from a Lung Cancer Screening | Saved By The Scan. American Lung Association. Retrieved October 17, 2022, from https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/saved-by-the-scan/resources/what-to-expect-from-lung-cancer-screening
- DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis. 2015 Dec;7(Suppl 4):S304-16.
- Mehta AC, Hood KL, Schwarz Y, Solomon SB. The Evolutional History of Electromagnetic Navigation Bronchoscopy: State of the Art. Chest. 2018 Oct;154(4):935-947.
FAQs
How is a bronchoscopy done?
A bronchoscopy is when a doctor or healthcare professional inserts a thin tube through the mouth or nose and into the lungs. A bronchoscope can be equipped with a light and small camera so the doctor can view the patient’s lungs and airways. This procedure is used to allow doctors to examine lesions and/or look for signs of cancer.
How long is recovery from a bronchoscopy?
A bronchoscopy is an outpatient procedure that typically takes 1-2 days to recover from. Patients may experience tiredness, a sore throat, and dry mouth. If these symptoms persist or become more severe, contact your doctor.